Embark on a scientific exploration with the activity series pogil answer key, an invaluable resource that unlocks the secrets of metal reactivity. This guide unveils the significance of the activity series in chemistry, empowering you to understand and predict the behavior of metals in various chemical processes.
The activity series, meticulously arranged in a tabular format, serves as a roadmap for metal reactivity, ranging from the most reactive to the least. Delve into the intricacies of displacement reactions, where metals engage in a competitive dance to replace one another, guided by the principles of the activity series.
Introduction
The activity series is a ranking of metals based on their reactivity. It is a useful tool for predicting the products of chemical reactions involving metals. The more reactive a metal is, the more easily it will react with other substances.
Understanding the reactivity of metals is important for a number of reasons. First, it can help us to predict the products of chemical reactions. Second, it can help us to design materials that are resistant to corrosion. Third, it can help us to understand the behavior of metals in biological systems.
Reactivity of Metals, The activity series pogil answer key
The reactivity of a metal is determined by a number of factors, including its atomic radius, its ionization energy, and its electronegativity. Metals with a small atomic radius, a low ionization energy, and a low electronegativity are more reactive than metals with a large atomic radius, a high ionization energy, and a high electronegativity.
The activity series can be used to predict the products of chemical reactions involving metals. For example, if a more reactive metal is added to a solution of a less reactive metal, the more reactive metal will displace the less reactive metal from the solution.
This is because the more reactive metal is more likely to lose electrons than the less reactive metal.
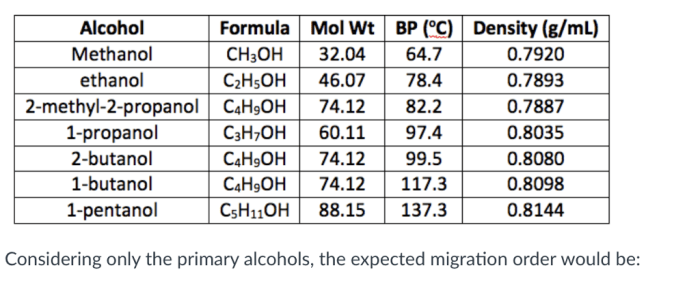
The Activity Series Table
The activity series of metals is a list of metals arranged in order of their reactivity. The more reactive a metal is, the more easily it will react with other substances.
The activity series can be used to predict the products of a reaction between two metals. If a more reactive metal is placed in contact with a less reactive metal, the more reactive metal will oxidize (lose electrons) and the less reactive metal will reduce (gain electrons).
Activity Series of Metals
| Metal | Reactivity |
|---|---|
| Potassium | Most reactive |
| Sodium | |
| Calcium | |
| Magnesium | |
| Aluminum | |
| Zinc | |
| Iron | |
| Nickel | |
| Tin | |
| Lead | |
| Hydrogen | |
| Copper | |
| Silver | |
| Gold | Least reactive |
Predicting Reactivity

The Activity Series is a valuable tool for predicting the reactivity of metals. It helps us understand how metals will react with other substances, such as acids or other metals.
One important concept related to the Activity Series is that of displacement reactions. A displacement reaction is a reaction in which a more reactive metal displaces a less reactive metal from a compound. For example, if you place a piece of iron in a solution of copper sulfate, the iron will displace the copper from the solution and form iron sulfate.
Using the Activity Series to Predict Reactivity
The Activity Series can be used to predict the reactivity of metals in several ways. First, it can be used to predict whether a metal will react with an acid. Metals that are more reactive than hydrogen will react with acids to produce hydrogen gas.
For example, iron will react with hydrochloric acid to produce hydrogen gas and iron chloride.
Second, the Activity Series can be used to predict whether a metal will react with another metal. A more reactive metal will displace a less reactive metal from a compound. For example, iron will displace copper from copper sulfate.
Applications of the Activity Series: The Activity Series Pogil Answer Key

The Activity Series is a practical tool with numerous applications in various fields. It plays a crucial role in corrosion prevention and battery design, among other applications.
In corrosion prevention, the Activity Series helps predict the reactivity of metals and their susceptibility to corrosion. By understanding the relative reactivity of different metals, engineers can select appropriate materials for specific applications, such as choosing corrosion-resistant metals for pipelines or storage tanks.
Battery Design
The Activity Series is essential in designing batteries. Batteries rely on electrochemical reactions between two dissimilar metals, known as the anode and cathode. The Activity Series guides the selection of suitable anode and cathode materials to achieve the desired voltage and current output.
For instance, in a lead-acid battery, lead is used as the anode, while lead dioxide is used as the cathode, based on their relative positions in the Activity Series.
Limitations of the Activity Series
The activity series provides a valuable tool for predicting the reactivity of metals, but it has certain limitations. These limitations arise from factors that can influence the reactivity of metals, such as temperature, concentration, and surface area.
The activity series assumes that the reactions occur under standard conditions (1 M concentration, 25 °C, and 1 atm pressure). However, changes in these conditions can affect the reactivity of metals. For example, increasing the temperature generally increases the reactivity of metals, while decreasing the concentration of the reactants can slow down the reaction.
Surface Area
The surface area of a metal can also affect its reactivity. A metal with a larger surface area has more atoms exposed to the reactants, which increases the chances of a reaction occurring. For example, finely divided metals (such as powders) are more reactive than their bulk counterparts because they have a larger surface area.
Conclusion
The Activity Series is a valuable tool for understanding the reactivity of metals and their behavior in chemical reactions. It allows us to predict the outcome of reactions involving different metals and to design experiments accordingly.
Understanding the reactivity of metals is crucial in various chemical processes, including corrosion, electroplating, and the production of batteries. By harnessing the knowledge of the Activity Series, scientists and engineers can optimize these processes, leading to improved efficiency, reduced costs, and enhanced safety.
Essential FAQs
What is the activity series?
The activity series is a ranking of metals based on their reactivity, arranged from most reactive to least reactive.
How can the activity series be used to predict reactivity?
The activity series can be used to predict the reactivity of metals in displacement reactions. A more reactive metal will displace a less reactive metal from a compound.
What are some limitations of the activity series?
The activity series does not take into account factors such as temperature, concentration, and surface area, which can affect the reactivity of metals.