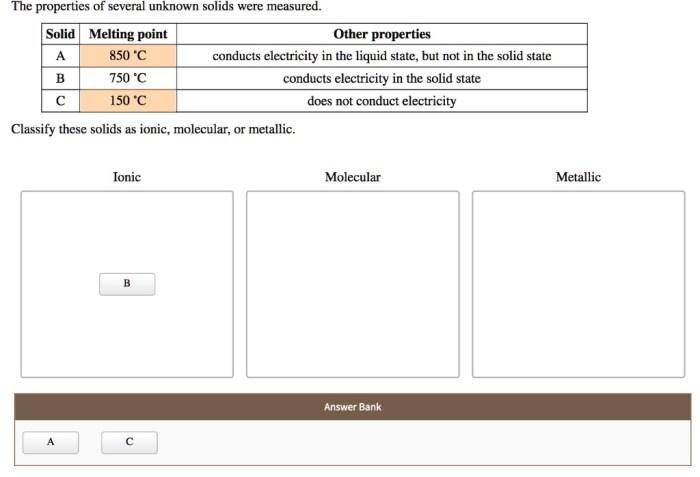
As the properties of several unknown solids were measured, a captivating journey into the realm of materials science unfolds. This discourse delves into the intricate relationships between the chemical composition and crystal structure of solids and their diverse physical, thermal, electrical, magnetic, and optical attributes.
Prepare to unravel the mysteries of these enigmatic substances as we embark on a quest for knowledge and understanding.
The exploration begins with an examination of physical properties such as density, hardness, and melting point, revealing the fundamental characteristics of solids. Thermal properties, including specific heat capacity and thermal conductivity, shed light on the heat transfer capabilities of these materials.
Delving deeper, electrical properties like electrical conductivity and dielectric constant unveil the ability of solids to conduct and store electrical energy.
Physical Properties
Solids possess distinct physical properties that characterize their behavior and response to external stimuli. These properties include density, hardness, and melting point.
Density refers to the mass of a solid per unit volume and is expressed in grams per cubic centimeter (g/cm³). It provides insights into the compactness of a solid’s structure.
Hardness measures the resistance of a solid to deformation or scratching. It is quantified using the Mohs scale, where diamond ranks highest at 10 and talc at 1.
Melting point represents the temperature at which a solid transitions into a liquid state. It indicates the strength of intermolecular forces within the solid.
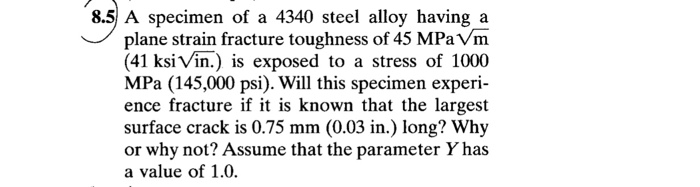
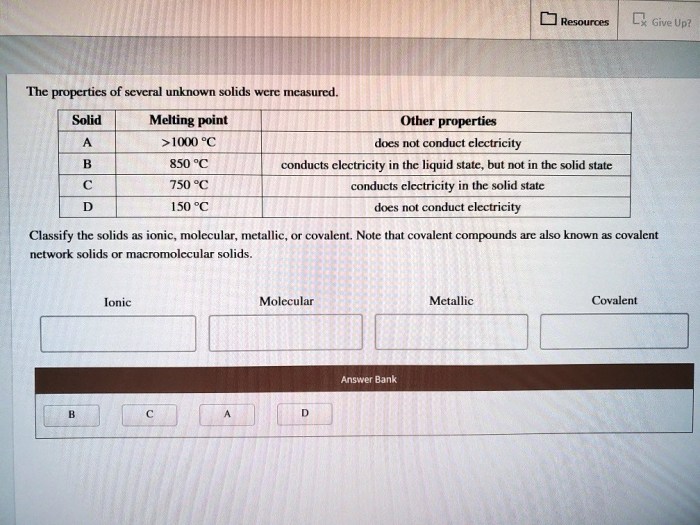
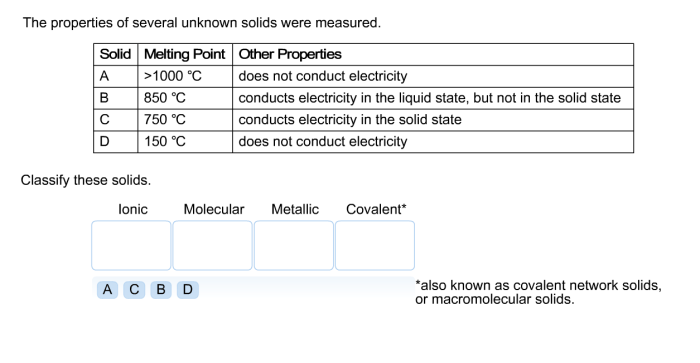
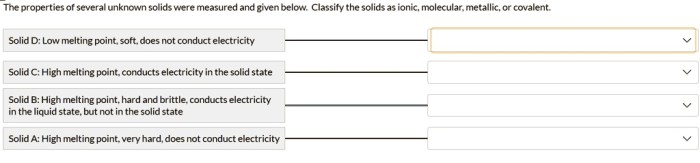
Relationship to Chemical Composition and Crystal Structure, The properties of several unknown solids were measured
The physical properties of solids are closely linked to their chemical composition and crystal structure.
Chemical composition determines the atomic constituents of a solid, which influences its density and hardness. For instance, metals tend to be denser and harder than non-metals.
Crystal structure refers to the arrangement of atoms or molecules within a solid. Different crystal structures, such as cubic, hexagonal, or amorphous, affect the melting point, density, and hardness of a solid.
Thermal Properties
Thermal properties of solids describe their response to heat. Key thermal properties include specific heat capacity and thermal conductivity.
Specific heat capacity measures the amount of heat required to raise the temperature of a unit mass of a solid by one degree Celsius. It indicates the ability of a solid to store thermal energy.
Thermal conductivity quantifies the rate at which heat flows through a solid. It is expressed in watts per meter-kelvin (W/m-K) and reflects the solid’s ability to conduct heat.
Relationship to Chemical Composition and Crystal Structure, The properties of several unknown solids were measured
The thermal properties of solids are influenced by their chemical composition and crystal structure.
Chemical composition affects the specific heat capacity, as different elements have varying heat capacities. For example, metals generally have higher specific heat capacities than non-metals.
Crystal structure impacts thermal conductivity. Solids with ordered crystal structures, such as metals, tend to have higher thermal conductivity than those with disordered structures.
Electrical Properties
Electrical properties of solids characterize their response to electric fields. Key electrical properties include electrical conductivity and dielectric constant.
Electrical conductivity measures the ability of a solid to conduct electric current. It is expressed in siemens per meter (S/m) and indicates the ease with which electrons can move through the solid.
Dielectric constant quantifies the ability of a solid to store electrical energy. It represents the ratio of the capacitance of a capacitor filled with the solid to the capacitance of the same capacitor filled with a vacuum.
Relationship to Chemical Composition and Crystal Structure, The properties of several unknown solids were measured
The electrical properties of solids are influenced by their chemical composition and crystal structure.
Chemical composition determines the number of free electrons available for conduction, which affects electrical conductivity. For instance, metals have a large number of free electrons, resulting in high electrical conductivity.
Crystal structure affects the dielectric constant. Solids with ordered crystal structures, such as ceramics, tend to have higher dielectric constants than those with disordered structures.
Magnetic Properties: The Properties Of Several Unknown Solids Were Measured
Magnetic properties of solids describe their response to magnetic fields. Key magnetic properties include diamagnetism, paramagnetism, and ferromagnetism.
Diamagnetism refers to the weak repulsion of a solid to a magnetic field. It is observed in materials with all electrons paired, resulting in no net magnetic moment.
Paramagnetism arises from the presence of unpaired electrons in a solid. These unpaired electrons align with an applied magnetic field, creating a weak attraction.
Ferromagnetism occurs in solids with unpaired electrons that align spontaneously, creating a strong magnetic field within the material.
Relationship to Chemical Composition and Crystal Structure, The properties of several unknown solids were measured
The magnetic properties of solids are influenced by their chemical composition and crystal structure.
Chemical composition determines the number of unpaired electrons, which affects the type of magnetic behavior. For example, iron has unpaired electrons, making it ferromagnetic.
Crystal structure affects the alignment of unpaired electrons. Solids with ordered crystal structures, such as magnetite, exhibit strong ferromagnetism.
Optical Properties
Optical properties of solids describe their interaction with light. Key optical properties include refractive index and absorption coefficient.
Refractive index measures the bending of light as it passes through a solid. It is expressed as a dimensionless number and indicates the speed of light in the solid relative to its speed in a vacuum.
Absorption coefficient quantifies the amount of light absorbed by a solid. It is expressed in inverse centimeters (cm⁻¹) and indicates the opacity of the solid.
Relationship to Chemical Composition and Crystal Structure, The properties of several unknown solids were measured
The optical properties of solids are influenced by their chemical composition and crystal structure.
Chemical composition determines the electronic structure of a solid, which affects its refractive index and absorption coefficient. For instance, solids with high electron densities tend to have higher refractive indices.
Crystal structure affects the propagation of light through a solid. Solids with ordered crystal structures, such as crystals, exhibit birefringence, where light is split into two beams with different polarizations.
Commonly Asked Questions
What is the significance of measuring the properties of solids?
Understanding the properties of solids is crucial for predicting their behavior in various applications, optimizing their performance, and designing new materials with tailored properties.
How can the properties of solids be measured?
A range of techniques are employed to measure the properties of solids, including density measurements, calorimetry, electrical conductivity measurements, magnetic susceptibility measurements, and optical spectroscopy.
What factors influence the properties of solids?
The properties of solids are primarily influenced by their chemical composition, crystal structure, and microstructure, which determine their atomic arrangement and bonding characteristics.