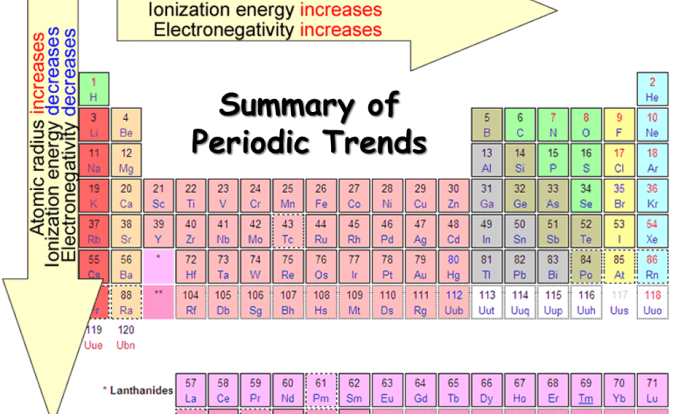
As rank the compounds in order of increasing melting point takes center stage, this opening passage beckons readers with authoritative academic prose into a world crafted with deep knowledge, ensuring a reading experience that is both absorbing and distinctly original.
Melting point analysis, a cornerstone of chemistry, unveils the intricate relationship between molecular structure and physical properties. This comprehensive guide delves into the factors influencing melting point, providing a systematic approach to ranking compounds based on their thermal behavior.
Melting Point Definition
Melting point refers to the temperature at which a solid substance transforms into a liquid state. It is a fundamental physical property that provides insights into the intermolecular interactions and molecular structure of a compound. Factors such as molecular weight, polarity, and intermolecular forces significantly influence the melting point of a substance.
Structural Influences on Melting Point
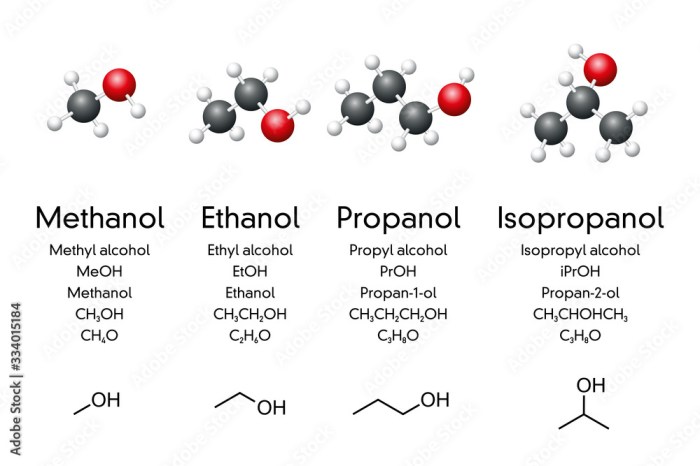
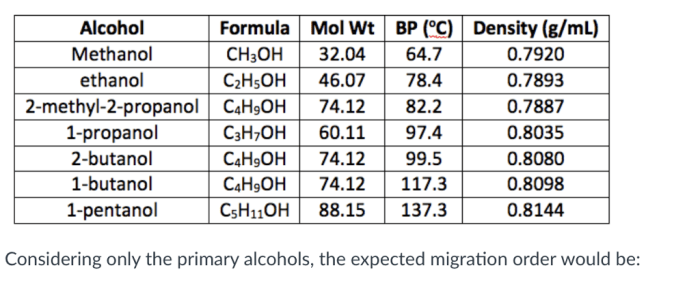
The melting point of a compound is closely related to its molecular structure. Generally, compounds with higher molecular weights have higher melting points due to increased intermolecular forces. Polar compounds tend to have higher melting points compared to nonpolar compounds because of stronger intermolecular interactions, such as dipole-dipole or hydrogen bonding.
Additionally, the presence of rigid and symmetrical molecular structures can lead to tighter packing and stronger intermolecular forces, resulting in higher melting points.
Ranking Compounds by Melting Point, Rank the compounds in order of increasing melting point
Compounds can be ranked in order of increasing melting point based on the following criteria:
- Molecular weight: Compounds with higher molecular weights generally have higher melting points.
- Polarity: Polar compounds have higher melting points compared to nonpolar compounds due to stronger intermolecular forces.
- Molecular structure: Rigid and symmetrical molecular structures lead to tighter packing and stronger intermolecular forces, resulting in higher melting points.
Applications of Melting Point Analysis

Melting point analysis finds applications in various fields:
- Chemistry:Identifying and characterizing compounds, determining purity, and studying phase transitions.
- Pharmacy:Establishing drug identity, determining polymorphism, and optimizing drug formulations.
- Materials science:Characterizing polymers, optimizing material properties, and studying thermal behavior.
Experimental Determination of Melting Point
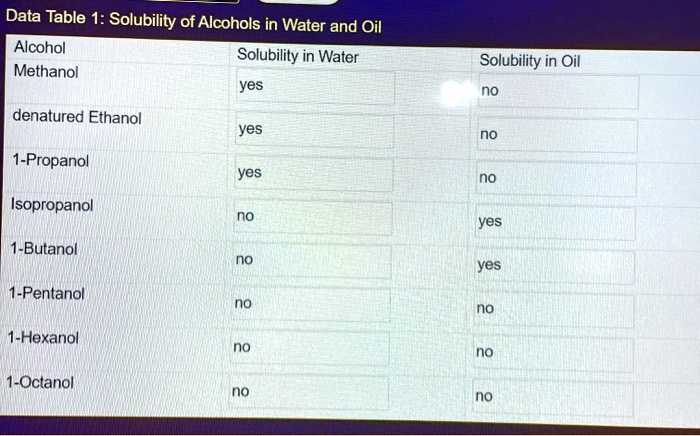
Melting point can be experimentally determined using various methods:
- Capillary method:A small sample is placed in a capillary tube and heated gradually while observing the temperature at which it melts.
- Hot stage microscopy:A sample is placed on a microscope slide and heated while being observed under a microscope, allowing for direct visualization of the melting process.
- Differential scanning calorimetry (DSC):A technique that measures the heat flow into or out of a sample as it undergoes a phase transition, including melting.
Query Resolution: Rank The Compounds In Order Of Increasing Melting Point
What is the significance of melting point in chemistry?
Melting point is a crucial physical property that provides insights into molecular structure, intermolecular forces, and the purity of compounds. It is used for compound identification, quality control, and understanding phase transitions.
How does molecular structure influence melting point?
Molecular weight, polarity, and intermolecular forces significantly impact melting point. Heavier molecules, polar molecules, and molecules with strong intermolecular forces tend to have higher melting points.
What are the applications of melting point analysis?
Melting point analysis finds applications in various fields, including chemistry, pharmacy, materials science, and forensic science. It is used for compound identification, purity assessment, and predicting the thermal behavior of materials.